Fe Cation Charge
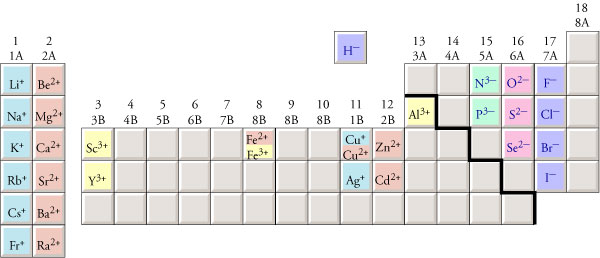
Cations & Anions Cations include everything from hydrogen, with a +1 positive charge, to aluminum, with a +3 positive charge. Anions include phosphate, nitrate, and other essential elements that hold a -1 or -2 charge. Table 1 shows the most common cations and anions along with their chemical formula and charge.
What is the charge on the iron cations in iron(II) chloride and iron (III) chloride, respectively?
1 Answer
- Examples of this enhancement include the charge-storage properties of certain cation-deficient oxides such as γ-MnO 2 and γ-Fe 2 O 3 relative to their defect-free analogues. The optimal cation-vacancy fraction is both material- and application-dependent because cation vacancies enhance some materials properties at the expense of others.
- Give either the name or formula (with the correct charge) for each of the cations.
Fe Cation Charge Formula
The Roman numerals after the name of the iron cation indicates the charge on the iron cation.
Explanation:
When naming ionic compounds which contain metal ions capable of forming more than one kind of cation, the Roman numeral after the metal's name indicates the charge.
Therefore, the iron cation in iron(II) chloride has a charge of
This video provides some additional examples of how to use Roman numerals when naming compounds.
In the image below, a solution of iron(III) chloride is on the left side and a solution of iron(II) chloride is on the right side.
Periodic Table With Ion Charges Labeled
Related questions
Carbon Ion Charge
Cation Names and Formulas Help
Metallic atoms hold some of their electrons relatively loosely, and as a result, they tend to lose electrons and form cations. In contrast, nonmetallic atoms attract electrons more strongly than metallic atoms, and so nonmetals tend to gain electrons and form anions. Thus, when a metallic element and a nonmetallic element combine, the nonmetallic atoms often pull one or more electrons far enough away from the metallic atoms to form ions. The positive cations and the negative anions then attract each other to form ionic bonds.
Carbon Charge
Predicting Monatomic Cation Charges The atoms of the noble gases found in nature are uncombined with other atoms. The fact that the noble gas atoms do not gain, lose, or share their electrons suggests there must be something especially stable about having 2 (helium, He), 10 (neon, Ne), 18 (argon, Ar), 36 (krypton, Kr), 54 (xenon, Xe), or 86 (radon, Rn) electrons. This stability is reflected in the fact that some metallic atoms form cations in order to get the same number of electrons as the nearest noble gas. See below.
| Monatomic Cation Names The names of monatomic cations always start with the name of the metal, sometimes followed by a Roman numeral to indicate the charge of the ion. For example, Cu+ is copper(I), and Cu2+ is copper(II). The Roman numeral in each name represents the charge on the ion and allows us to distinguish between more than one possible charge. Notice that there is no space between the end of the name of the metal and the parentheses with the Roman numeral. If the atoms of an element always have the same charge, the Roman numeral is unnecessary (and considered to be incorrect). For example, all cations formed from sodium atoms have a +1 charge, so Na+ is named sodium ion, without the Roman numeral for the charge. The following elements have only one possible charge, so it would be incorrect to put a Roman numeral after their name.
Although silver can form both +1 and +2 cations, the +2 is so rare that we usually name Ag+ assilver ion, not silver(I) ion. Ag2+ is named silver(II) ion. We will assume that all of the metallic elements other than those mentioned above can have more than one charge, so their cation names will include a Roman numeral. For example, Mn2+ is named manganese(II). We know to put the Roman numeral in the name because manganese is not on our list of metals with only one charge. Polyatomic Cation Names There is only one common polyatomic ion. Its formula is NH4+, and its name is ammonium. |